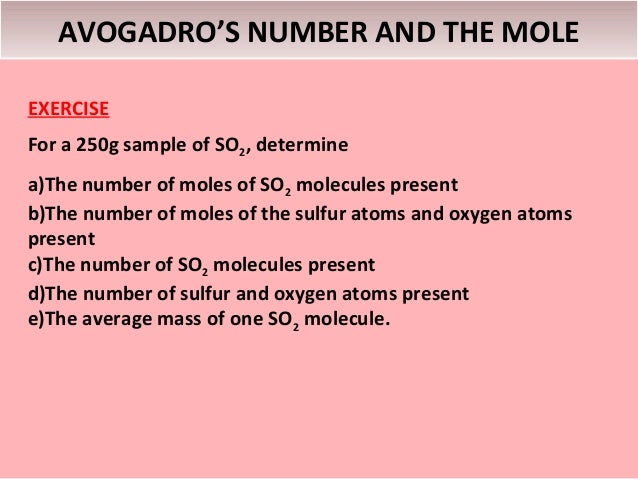
Avogadro's number is the number of particles in one mole of any substance. Its numerical value is 6.02225 × 1023. One mole of oxygen gas contains 6.02 × 1023molecules of oxygen, while one mole of sodium chloride contains 6.02 × 1023sodium ions and 6.02 × 1023 chloride ions. Avogadro's number is used extensively in calculating the volumes, masses, and numbers of particles involved in chemical changes.
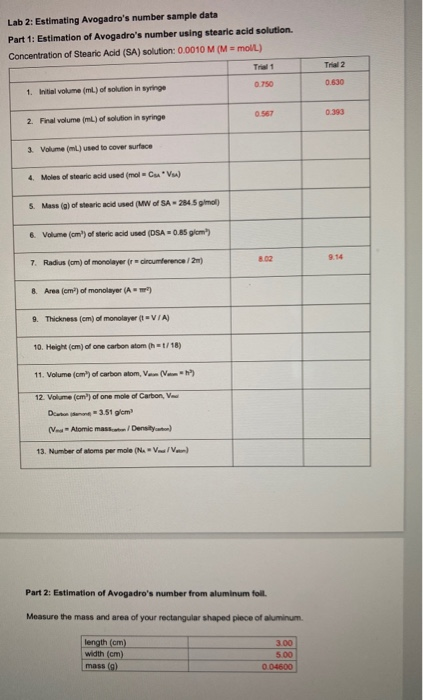
The Avogadro constant or (the Avogadro number earlier) is the number of elementary units in one mole of any substance. The Avogadro constant is denoted as NA. It has the dimension of the reciprocal amount of substance (mol −1). The approximate value of NA is 6.022 × 10 23 mol −1. Avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. The units may be electrons, atoms, ions, or molecules, depending on the nature of the substance and the character of the reaction (if any). See also Avogadro’s law. This number is called Avogadro’s number (N A), in honor of the Italian scientist Amedeo Avogadro. The currently accepted value is The currently accepted value is. Generally, we round Avogadro’s number to 6.022 × 10 23. Number of atoms per mole (Ne - Yo Yow 7.159x103 7.224x10 Part 2: Estimation of Avogadro's number from aluminum foil. Measure the mass and area of your rectangular shaped piece of aluminum. Length (cm) width (cm) mass (8) ) 3.00 5.00 0.04600 Data Part 1 1. Was the estimation of Avogadro's number using steric acid accurate?
The concept that a mole of any substance contains the same number of particles arose out of research conducted in the early 1800s by the Italian physicist Amedeo Avogadro (1776-1856). Avogadro based his work on the earlier discovery by Joseph Gay-Lussac that gases combine with each other in simple, whole-number ratios of volumes. For example, one liter of oxygen combines with two liters of hydrogen to make two liters of water vapor.
1 Avogadro Number

Avogadro argued that the only way Gay-Lussac's discovery could be explained was to assume that one liter of any gas contains the same number of particles as one liter of any other gas. To explain the water example above, he further hypothesized that the particles of at least some gases consist of two particles bound together, a structure to which he gave the name molecule.
The question then becomes, 'What is this number of particles in a liter of any gas?' Avogadro himself never attempted to calculate this. Other scientists did make that effort, however. In 1865, for example, the German physicist J. Loschmidt estimated the number of molecules in a liter of gas to be 2.7 × 1022. The accepted value today is 2.69 × 1022.


What Is Avogadro Number
For all elements and compounds, not just gases, a given weight must contain a certain number of atoms or molecules. A weight (in grams) equal to the atomic or molecular weight of the substance-that is, one mole of any element or compound-must contain the same number of atoms or molecules, because there is always a constant relationship between atomic weights and grams. (One atomic mass unit = 1.66 × 10-22 g.) The number of atoms or molecules in one mole of an element or compound has been named Avogadro's number, in honor of his realization about the numbers of particles in gases. As stated above, that number has been determined to be 6.0225 × 10 23.
See also Atomic weight.
Additional topics
Science EncyclopediaScience & Philosophy: A-series and B-series to Ballistic Missiles - Categories Of Ballistic Missile
